Stainless steel has been used as the standard construction material for equipment at wastewater plants since the 1960s. Although it does not readily corrode or rust, stainless steel components in water and wastewater environments are still susceptible to corrosion because they are exposed to high levels of chlorides and sulfides. These elements cause corrosion through exposed and embedded free iron on the surface of the stainless steel. Embedded iron on the surface of the stainless steel is caused by heat procedures used in the manufacturing process. One of the methods used to protect the stainless steel in manufactured equipment is called “pickling.” This technical brief explains that process.
Stainless steel: Corrosion resistant but not corrosion-proof
Stainless steel uses chromium to resist corrosion. The chromium reacts with the oxygen in the air, forming a thin film of chromium oxide on its surface. Chromium oxide is inert, or “passive,” making the stainless steel protected against rust and corrosion. Anything that causes the chromium oxide protective film to break down will promote corrosion in stainless steel. Several common factors include:
 pads, wire brushes and scrapers. These tools should be used with care, and the surface of the stainless steel should be treated afterward to restore the chromium oxide layer.
pads, wire brushes and scrapers. These tools should be used with care, and the surface of the stainless steel should be treated afterward to restore the chromium oxide layer.In wastewater plants, chloride levels in the influent pose the greatest risk for corrosion in stainless steel. Some of the biggest contributors are water softeners, road salt, and fertilizers; water softeners being the largest single source of chlorides to a wastewater stream. Higher chloride levels can result in crevice corrosion and pitting.
 Crevice Corrosion occurs when two mating surfaces are not properly treated prior to being mated. The gap or crevice can be formed between two metals or a metal and non-metallic material. Water becomes trapped, creating a ripe area for corrosion.
Crevice Corrosion occurs when two mating surfaces are not properly treated prior to being mated. The gap or crevice can be formed between two metals or a metal and non-metallic material. Water becomes trapped, creating a ripe area for corrosion.
 Pitting Corrosion is a breakdown of the chromium oxide layer followed by localized corrosion that produces pits, which may cause perforation of a vessel or pipework.
Pitting Corrosion is a breakdown of the chromium oxide layer followed by localized corrosion that produces pits, which may cause perforation of a vessel or pipework.
All stainless steel is not equal
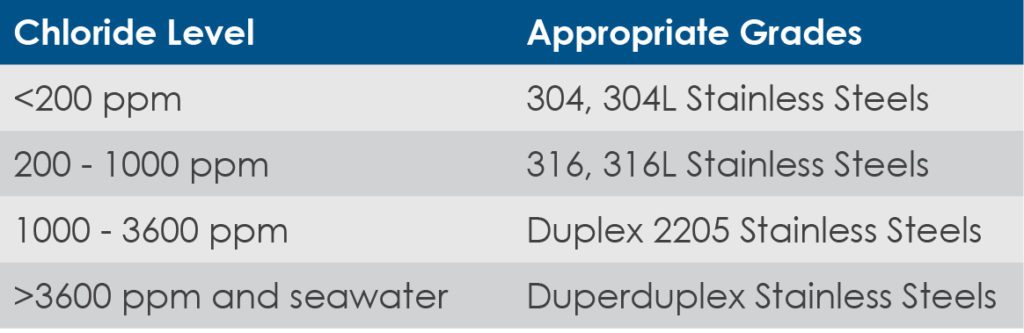
Although all stainless steel types share similar properties, 316 stainless steel includes molybdenum in the manufacturing process, making it more resistant to chloride corrosion and more acidic environments.
It is important to understand the levels of chloride in the treatment process and select the appropriate grade of stainless steel.
Pickling: The preferred method for protecting stainless steel ASTM A380 is an international standard that outlines the best ways to treat stainless steel with a pickling process. Pickling is the removal of oxide films that form where the stainless steel’s surface chromium level has been reduced because of hot-forming operations, such as welding. These oxide films contain embedded iron, heat tint and weld scale, and are a major cause of corrosion on stainless steel. That is why stainless steel that has been affected by manufacturing processes should always be treated using a pickling process.
ASTM A380 is an international standard that outlines the best ways to treat stainless steel with a pickling process. Pickling is the removal of oxide films that form where the stainless steel’s surface chromium level has been reduced because of hot-forming operations, such as welding. These oxide films contain embedded iron, heat tint and weld scale, and are a major cause of corrosion on stainless steel. That is why stainless steel that has been affected by manufacturing processes should always be treated using a pickling process.
Pickling is a dual process which uses two acid types: Hydrofluoric acid, which removes the scale or embedded iron, and Nitric acid, which expedites a naturally occurring attribute in stainless steel called passivation. Passivity is a condition where chromium creates a protective surface that doesn’t allow outside influences to interact with the stainless steel iron to cause corrosion.
Pickling Process Steps:
1. Prior to pickling, the stainless steel must be cleaned of impurities such as dirt, grease, and oil.
2. Rinse cleaning residue.
3. Descale the stainless steel by removing the oxide scale from the surface using hydrofluoric acid.
4. Remove residual acid.
5. Apply the pickling process using one of three application types:
 When you are evaluating new equipment for your facility, be sure to evaluate not only its features and functionality, but also the material that goes into it, as well as the manufacturer. Consider these facts:
When you are evaluating new equipment for your facility, be sure to evaluate not only its features and functionality, but also the material that goes into it, as well as the manufacturer. Consider these facts:
4750 118th Avenue North Clearwater, Florida 33762 USA Phone: +1 (813) 818-0777 Fax: (813) 818-0770
Copyright ©2025 Hydro-Dyne Engineering. All Rights Reserved. | Privacy Policy | Terms of Use